Reduction of Acid Halides To Aldehydes With LiAlH(OtBu)3 or Rosenmund’s Catalyst
- Acid halides can be reduced to aldehydes through use of the bulky reducing agent lithium aluminium (tri t-butoxy)hydride
- The mechanism for this reaction is a fairly straightforward nucleophilic acyl substitution; addition of hydride to the carbonyl followed by elimination of halide.
- Acid halides can also be reduced to aldehydes using Pd-C / barium carbonate with hydrogen, otherwise known as Rosenmund’s catalyst

Table of Contents
- Lithium Tri-t-butoxyaluminum hydride, LiAlH(O-tBu)3
- Reduction of Acid Halides by LiAlH(O-tBu)3
- The Mechanism for Reduction of Acid Halides to Aldehydes by LiAlH(OtBu)3
- Partial Reduction of Acid Halides to Aldehydes with Pd-BaCO3/H2 (Rosenmund Catalyst)
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Lithium Tri tert-butoxyaluminum hydride LiAlH(O-t-Bu)3
Lithium aluminum hydride (LiAlH4) is a useful reducing agent, but it’s kind of a sledgehammer as far as reagents go.
If you want to reduce every C=O bond in sight, along with alkyl halides and epoxides, by all means use LiAlH4.
If you want a reagent that will be a bit more selective, it’s possible to modify the structure of LiAlH4 such that only the most reactive C=O bonds are reduced.
One example is the reagent formed when LiAlH4 is treated with three equivalents of t-butanol to give lithium tri (tert-butoxy) aluminum hydride, LiAlH(Ot-Bu)3. [Note 1]
Lithium Tri-tert-butoxyaluminum Hydride (LiAlH(Ot-Bu)3)
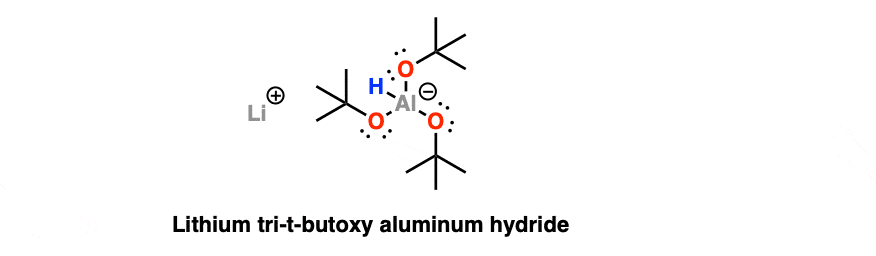
This reagent has two advantages. First of all, it only has one hydride to give, unlike LiAlH4, so it’s a lot easier to control the reaction using the proper stoichiometry.
Secondly, those big bulky tert-butoxy groups (that’s -OC(CH3)3) help to modulate (i.e. slow down) the reactivity of the reagent. They’re kind of like a fat suit around aluminum that ensure that the hydride can’t fit into tight spaces.
2. Reduction Of Acid Chlorides To Aldehydes By LiAlH[OC(CH3)3)]3
So what’s it used for? One big thing. It will reduce acid chlorides to aldehydes, and stop there.
That is extremely useful, because aldehydes are easily reduced to alcohols. So if you use just one equivalent of the reagent, you’ll end up with one equivalent of the aldehyde, (so long as the reaction mixture is kept cold). And aldehydes can themselves be used in all kinds of useful applications.
This serves as a way to indirectly reduce carboxylic acids to aldehydes. You can convert the carboxylic acid to an acid chloride using something like SOCl2 or PCl3, and then reduce the acid chloride to the aldehyde with LiAlH[OC(CH3)3)]3
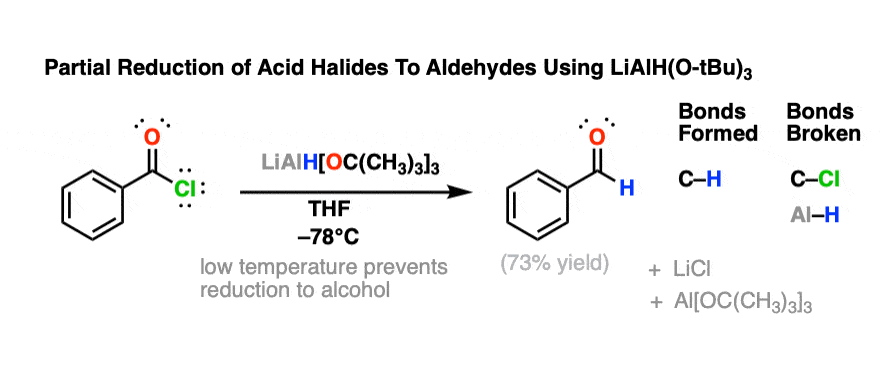
What is the advantage of using LiAlH(Ot-Bu)3 , relative to LiAlH4?
- First of all, it saves us a step. We could use LiAlH4 to reduce the acid halide down to the alcohol, and then oxidize the primary alcohol up to the aldehyde using a reagent like PCC, Dess-Martin periodinane, or the Swern oxidation. (See post: Oxidation of Alcohols). But that’s an extra step. Since yields are never 100%, that means that we’ll risk losing some of our precious product along the way.
- Secondly, it’s more selective. If we have a molecule with multiple functional groups (such as esters or lactones) along with our acid halide, then those will be reduced by LiAlH4 as well. However, if we use LiAlH(Ot-Bu)3, only the acid halide will be reduced.
3. The Mechanism For Reduction Of Acid Chlorides To Aldehydes By LiAlH(Ot-Bu)3
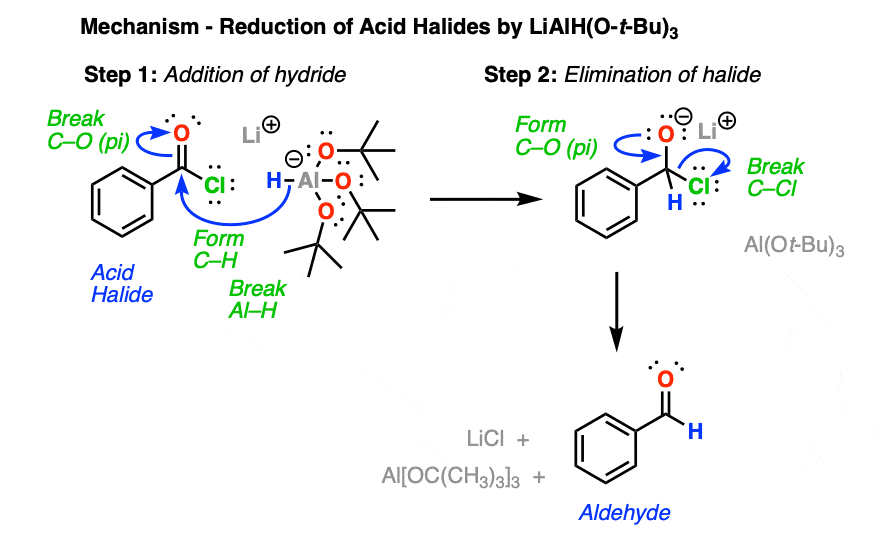
So how does it work? It’s pretty straightforward actually; another example of a nucleophilic acyl substitution reaction using the addition-elimination mechanism (See post: Nucleophilic Acyl Substitution)
The first step is addition of hydride to the carbonyl of the acid chloride, resulting in a tetrahedral intermediate (See post: Nucleophilic Addition).
This is followed by elimination of the best leaving group (choride) resulting in a new aldehyde.
So long as you are careful about employing only one equivalent of the reductant, the reaction should result in the formation of an aldehyde.
4. Partial Reduction of Acid Chlorides To Aldehydes With Rosenmund’s Catalyst
Another method for formation of aldehydes from acid halides employs a hydrogenation catalyst Pd-BaCO3 (or Pd-BaSO4) and H2.
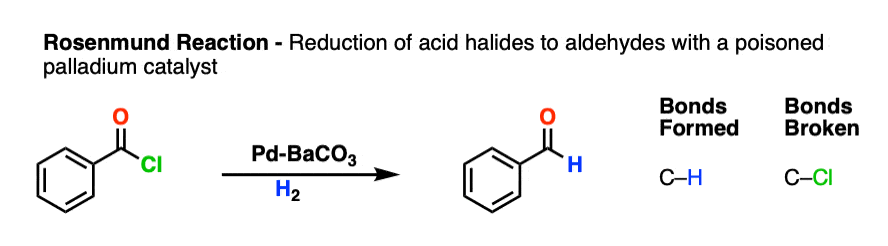
This is known as the Rosenmund reaction. The purpose of barium is to “poison” the palladium catalyst and make it less reactive, similar to the Lindlar catalyst.
Notes
Note 1. LiAlH(O-t-Bu)3 is commercially available as a solid or as a solution in THF or diglyme. It can also be prepared directly from LiAlH4 by adding three equivalents of t-BuOH. [Ref]
Note 2. According to reference 7, LiAlH(O-t-Bu)3 is a stronger reducing agent than NaBH4 but a significantly weaker reducing agent than LiAlH4.
Note 2. LiAlH(O-tBu)3 will also reduce aldehydes and ketones to alcohols, although it will not reduce esters, lactones, epoxides, nitriles, or alkyl halides.
Aldehydes can be reduced preferentially in the presence of ketones. [Ref]
Note 3. You can read about the chemistry of LiAlH(Ot-Bu)3 and more than 80 other reagents in undergraduate organic chemistry in the “Organic Chemistry Reagent Guide”, available here as a downloadable PDF.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- Über eine neue Methode zur Darstellung von Aldehyden. 1. Mitteilung Karl W. Rosenmund Ber. 1918, 51 (1), 585-593 DOI: 10.1002/cber.19180510170 The first paper by Karl Rosenmund on what is now called the Rosenmund reduction. This is basically a hydrogenolysis of an acyl chloride with H2 and Pd-BaCO3, called the Rosenmund catalyst.Among his numerous contributions to organic chemistry, Nobel Laureate Prof. H. C. Brown (Purdue) also developed aluminum reagents for this transformation, as shown below:
- LITHIUM TRI-t-BUTOXYALUMINOHYDRIDE-A NEW REAGENT FOR CONVERTING ACID CHLORIDES TO ALDEHYDES Herbert C. Brown and Richard F. McFarlin Journal of the American Chemical Society 1956, 78 (1), 252-252 DOI: 10.1021/ja01582a072
- A New Aldehyde Synthesis—The Reduction of Acid Chlorides by Lithium Tri-t-butoxyaluminohydride Herbert C. Brown and B. C. Subba RaoJournal of the American Chemical Society 1958, 80 (20), 5377-5380 DOI: 10.1021/ja01553a014
- Exceptionally facile reduction of acid chlorides to aldehydes by sodium tri-tert-butoxyaluminohydride Jin Soon Cha and Herbert C. Brown The Journal of Organic Chemistry 1993, 58 (17), 4732-4734 DOI: 10.1021/jo00069a043
- An effective one-pot conversion of acid chlorides to aldehydes and ketones Jae Kyo Park, Won Kyu Shin, Duk Keun An Tetrahedron Letters, Volume 54, Issue 24, 12 June 2013, Pages 3199-3203 DOI:10.1016/j.tetlet.2013.04.041 A variant of Prof. Brown’s method using DIBAL-H + morpholine for acid chloride reduction. The advantage is that both these reagents are readily available.
- LACTONE OF PREGNOIC ACID K. Heusler, P. Wieland, and Ch. Meystre Organic Syntheses,1965, 45, 57
- Selective Reductions. III. Further Studies of the Reaction of Alcohols with Lithium Aluminum Hydride as a Route to the Lithium Alkoxyaluminohydrides Herbert C. Brown and Charles J. Shoaf Journal of the American Chemical Society 1964 86 (6), 1079-1085 DOI: 10.1021/ja01060a023 In this paper, the relative power of various reducing agents is found to be LiAlH4 > LiAlH(OCH3)3 > LiAlH(O-t-Bu)3 > NaBH4


